by Dan Roberts

A great deal has happened in an amazingly short time in the field of stem cell transplantation for treatment of retinal disease. This article overviews the first seven years and briefly summarizes the most important research. By no means comprehensive, it is intended to provide a helpful perspective for both doctors and patients and serve as a starting point for further study.
Stem cells are undeveloped structures which are able to develop into any of the nearly 220 cell types that make up the human body, and which can theoretically reproduce themselves infinitely. Discovery of progenitor cells in the eyes of adult rodents generated research in the area of retinal cell transplantation.
2000
This discovery was reported at a meeting in 2000 of the Association for Research in Vision and Ophthalmology (ARVO) by Dr. Derek van der Kooy (University of Toronto) and Dr. Iqbal Ahmad (University of Nebraska). The researchers found that stem cells have certain characteristics of photoreceptor cells, but that further study was needed to determine if they could be fully functioning as such.
Dr. Michael Young (Schepens Eye Research Institute, Boston) added to this knowledge, reporting on his discovery that transplanted cells from a mouse retina were able to reproduce, and that some of them contained the photoreceptor-specific protein, rhodopsin, which initiates phototransduction.1
In addition, transplanted neural progenitor cells were found to be capable of responding to injury cues in the mature central nervous system by differentiating into cells that could take on the job of retinal neurons. Other encouraging research from Kyoto University also showed that transplanted stem cells from rodents seem to be able to form nerve synapse connections.2
2002
In August 2002, scientists at The Scripps Research Institute (TSRI) in La Jolla, California reported success in forming new retinal blood vessels in mice with ocular disease. The process uses pluripotent adult stem cells, which are derived from bone marrow and injected into the vitreous of the eyeball. When in place, these cells develop into endothelial cells and–in concert with astrocytes–form the lining of the new blood vessels. In the mouse models, this process stopped the progression of macular degeneration.3
Martin Friedlander, M.D., Ph.D. (Associate Professor, Department of Cell Biology; Chief of Retina Service, Division of Ophthalmology, Department of Surgery TSRI) was the leader of the study. According to Dr. Friedlander, not only could adult bone marrow stem cells be used to form new vessels, but they could also be used to deliver powerful antiangiogenic drugs to prevent neovascularization. This was promising news to people with neovascular macular degeneration.
2004
On September 23, 2004, Advanced Cell Technology (Alameda, California) announced that they had engineered human embryonic stem cells which could be used to repair a damaged retina.4 The research team worked with stem cells taken from human embryos made by another team at Harvard University and coaxed them to form retinal cells. Those cells were clearly defined as resembling rod and cone photoreceptors. Dr. Robert Lanza (Scientific Director) said the results illustrate the need to use cloning technology to eliminate the risk of rejection by the patient’s immune system.
A month later, in October 2004, Derek Van der Kooy (Department of Medical Biophysics, University of Toronto, Ontario, Canada ) announced to the National Academy of Sciences that stem cells from donated human retinas were cultured in the laboratory and then transplanted into the healthy retinas of young mice. After four weeks, most of the cells had migrated to the photoreceptor layer of the new retinas and successfully differentiated themselves into the cells necessary for sight.
In November 2004, California became the first state to circumvent the federal government’s restriction on funding for stem cell research5 by passing Proposition 71 with a majority vote of 69%. This allowed nearly three billion dollars to be put aside for stem cell research in that state over the next 10 years.
In the same month, scientists from Harvard’s Schepens Eye Research Institute successfully, and for the first time, improved the vision of mice with transplanted stem cells.6 Progenitor cells were obtained from day-old mice and grafted into the degenerating retinas of mature mice. The transplanted cells were then seen to develop into mature neurons.
The researchers observed “rescue of cells in the outer nuclear layer (ONL), along with widespread integration of donor cells into the inner retina, and recipient mice showed improved light-mediated behavior compared with control animals.” The procedure had preserved existing cells and restored health to those that were degenerating–a major step toward the therapeutic use of stem cells for people with all forms of retinal degeneration.
2006
In April 2006, a research team at the Dr Rajender Prasad Centre for Ophthalmic Sciences, All India Institute of Medical Sciences (AIIMS), reported that 50 patients severely affected by age-related macular degeneration or retinitis pigmentosa showed significant improvement in vision after one month of injecting stem cells, and that there was further improvement after a gap of three months.7 The research team, headed by Dr. Atul Kumar (Professor of Ophthalmology) used autologous bone marrow derived stem cells and injected them into a loose tissue near the cornea. Follow-ups were then done after one, three, six and 12 months.
Later that year, on August 14, 2006, Tom Reh (Professor of Biological Structure, University of Washington) said that if stem cell research at the UW and other institutions continues to be successful, the first human tests could begin by 2009.8 Led by Reh, the UW team used a mix of growth factors to coax embryonic cells into becoming retinal cells. Previous research had been conducted with mouse stem cells, but this was the first use of human stem cells using the technique for the retina.
After growing the embryonic cells in the lab for several weeks, the researchers first placed them in growth factors important to head development in humans and mice. They then added another factor that other scientists have found leads to large eye development in frogs. That combination stimulated the embryonic cells to become retinal progenitor cells. The development occurred in two weeks, ab out twice as fast as during normal development in the uterus. Finally, when the scientists mixed the new cells with damaged mouse retinal tissue, the cells replaced key structures, namely cones, rods, and amacrine cells. The team then began injecting the new cells into the eyes of retina-damaged mice, measuring nerve reactions to see whether there was actual vision improvement.9
On September 21, 2006, Raymond D. Lund (John A. Moran Eye Center, University of Utah, Salt Lake City) and Robert Lanza (Advanced Cell Technology) reported that cells grown from human embryonic stem cells slowed vision loss when injected into the eyes of rats with a disease similar to macular degeneration.10
The researchers achieved the transformation in all 18 stem cell lines they worked with, proving their approach could consistently produce the crucial pigment cells. Then they injected about 20,000 cells per eye into the retinas of 14 rats. Eight control rats received eye injections without any cells. Forty days after treatment, the team found that the treated rats were twice as responsive to flashes of light as the untreated ones, which by then were going blind. A separate test showed that the treated rats had twice the visual acuity of the untreated rats nearly three months after treatment. Microscopic examination of the retinas at autopsy showed that the treated eyes had healthy photoreceptor layers 5 to 7 cells thick, while the untreated eyes had an average thickness of just one cell. (Healthy rats have layers 10 to 12 cells thick.) None of the cells divided abnormally or grew into tumors.11
2007
Researchers continue to look for ways to avoid harming embryos during the production of new stem cell lines. Sources of adult cells, which sidestep the ethical problems, are bone marrow, brain tissue and umbilical cord blood. Some researchers, however, argue that adult cells are more difficult to produce in large quantities and that they may eventually lose their potency.
On January 7, 2007, researchers at the Institute for Regenerative Medicine at Wake Forest University School of Medicine discovered yet another potential source of embryonic stem cells in the womb’s amniotic fluid.12 These cells appear to be almost as malleable as those in the embryo itself, and the advantage would be that harvesting them would be harmless. Several more years of study are needed to assess their application in humans.
On another front, Advanced Cell Technology has been developing a technique for harmlessly removing a blastomere from an eight-cell human embryo (see photo). On June 2007, they issued a press release announcing successful production of a human embryonic stem cell line (hESC) using that method.13
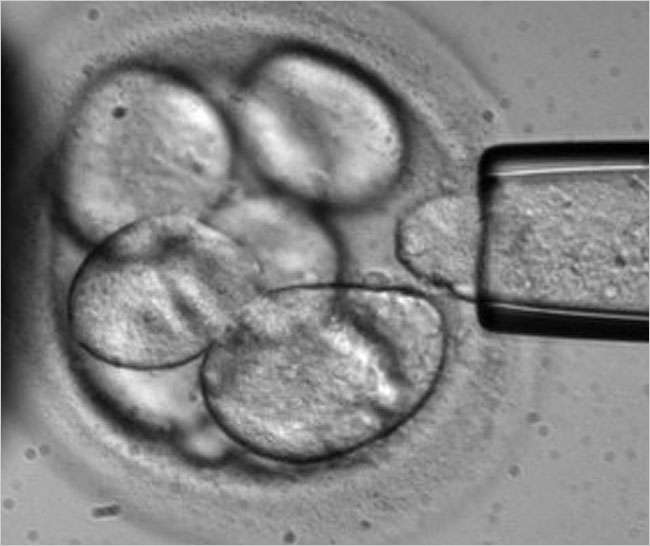
Biopsy of a human blastomere
(Photo courtesy of Advanced Cell Technology)
Other research is taking place in the United Kingdom at the University College London, Moorfields Eye Hospital and Sheffield University, in a cooperative effort called the London Project to Cure Blindness.14 Doctors at Moorfields have had some success with human subjects using adult stem cells from the patients’ own eyes. Embryonic cells, however, have been shown by the Sheffield scientists to be more malleable and easier to transplant than adult stem cells. Laboratory-grown cells from the blastocyst of a 5-day old embryo require only one injection (a 45-minute procedure), whereas the Moorfields experiments have taken two hours and two surgical procedures. This protocol would be very expensive and impractical in general practice, so embryos are being used at Sheffield, and that will take a little longer to get into human trials. Moorfields scientists are also studying the potential use of Müller glial cells from the patient’s eyes.15 These would be grown in vitro and transplanted back into the eye–a treatment that may be viable in as little as five years.
In late 2007, two research groups described a method of creating induced pluripoint stem cells by inserting master regulator genes into the chromosomes of human skin cells. The altered cells appear to behave like embryonic stem cells, in that they might be capable of changing into any one of the 220 types of cells in the human body. This could eventually eliminate the need for using human embryos for research. The results were to be published in Cell by Shinya Yamanaka (Kyoto University and the Gladstone Institute of Cardiovascular Disease in San Francisco), and in Science by James A. Thomson et al (University of Wisconsin).
Four genes were used to reprogram the human skin cells, all of which act to turn other genes on or off, essentially reprogramming the cells into which they are introduced. A current drawback, however, is that one of the genes has a 20 percent risk of causing cancer. This means that, until the problem is solved, the stem cells created would not be suitable for replacement in people with degenerative cell diseases such as diabetes and macular degeneration. More study is needed, also, to determine if the reprogrammed cells are indeed the same as those from embryos. If that can be confirmed, destruction of embryos and donation of human eggs would no longer be necessary for ongoing stem cell research.
Also in late 2007, Prof. Luis E. Abad, Head of Vitreoretinal Unit of COAT-vision, and Director of CERI, Murcia, Spain, announced the outcome of a study performed to demonstrate the changes resulting from implanting stem cells into eyes of rabbits with chorioretinal tissue injury induced by laser diode (Highlights of Ophthalmology, December 2007). A recovery of 90% of the injury was reported at the healing stage, as the transplanted cells survived and migrated toward the damaged areas of the retina and transformed into the rabbit’s own epithelial cells.
Whether or not the ethical issues will be resolved, and whether the developments will come from government or private sectors, stem cell transplantation as a retinal treatment will likely be a reality. Meanwhile, it is important to emphasize to the patient that it is only a treatment: definitely promising, but not the cure we all hope for. That, we must realize, is a few more miles down the road.
_________________________
References
1 Michael Young. Molecular and Cellular Neuroscience (Academic Press, September 2000)
2 Toda, H., et al. Neurons Generated from Adult Rat Hippocampal Stem Cells Form Functional Glutamatergic and GABAergic Synapses in Vitro (Experimental Neurology, Vol 165, Number 1, September 2000 , pp. 66-76)
3 “Bone marrow-derived stem cells target retinal astrocytes and can promote or inhibit retinal angiogenesis” (Nature Medicine, September 2002)
4 Cloning and Stem Cells (Vol 6, p 217, September 2004)
5 In August 2001, President Bush had approved government funding for research using embryonic stem cell lines, but only those which are already in existence.
6 Henry J. Klassen, et al. Multipotent Retinal Progenitors Express Developmental Markers, Differentiate into Retinal Neurons, and Preserve Light-Mediated Behavior (Investigative Ophthalmology and Visual Science. 2004;45:4167-4173.)<
7 Atul Kumar, et al. Use of Autologous Bone Marrow Derived Stem Cells for Rehabilitation of Patients with Dry Age Related Macular Degeneration and Retinitis Pigmentosa: Phase-1 Clinical Trial. (Indian Journal of Medical & Paediatric Oncology, Vol 26, Suppl. 2005)
8 Warren King, "Stem-cell procedure promising" (Seattle Times:August 14, 2006)
9 Tom Reh and Deepak Lamba. (Proceedings of the National Academy of Sciences-online version. August 2006)
10 Rick Weiss. "Stem Cell Experiments Slow Vision Loss in Rats" (Washington Post. September 21, 2006)
11 Robert Lanza and Raymond D. Lund (Cloning and Stem Cells. September 2006; Vol 8, Number 3)
12 Anthony Atala, et al. Isolation of amniotic stem cell lines with potential for therapy (Nat Biotechnology. Jan 7, 2007)
13 www.advancedcell.com/press-release/advanced-cell-technology-develops-first-human-embryonic-stem-cell-line-without-destroying-an-embryo
14 www.thelondonproject.org
15 Jean M. Lawrence, et al. Mio-M1 Cells and Similar Müller Glial Cell Lines Derived from Adult Human Retina Exhibit Neural Stem Cell Characteristics (Stem Cells. Vol 25, Number 8)
© Dan Roberts, 2007