CONCERTO clinical trial is seeking participants to evaluate the new investigational SING IMT
Long-time members of our AMD community may remember FDA approval in 2010 of a device from Samsara Vision (formerly VisionCare, Inc.) called the Implantable Miniature Telescope (IMT).
This device is a tiny telescope which provides precision images, magnification of up to 3 times, and a 24º field of view (limited peripheral vision in implanted eye). The device is put into one eye in an outpatient surgical procedure under local anesthesia. It has since been given to more than 600 patients (1) who have vision loss from age-related macular degeneration (AMD).
We now want to share an investigational, new version of the IMT offered under the brand name SING IMTTM (Smaller-Incision New-Generation Implantable Miniature Telescope).
The tiny telescope is inserted through a cut in the cornea of the eye. This new version of the IMT can be folded and inserted through a surgical tool. This allows for a smaller cut in the eye and a less complicated surgery.
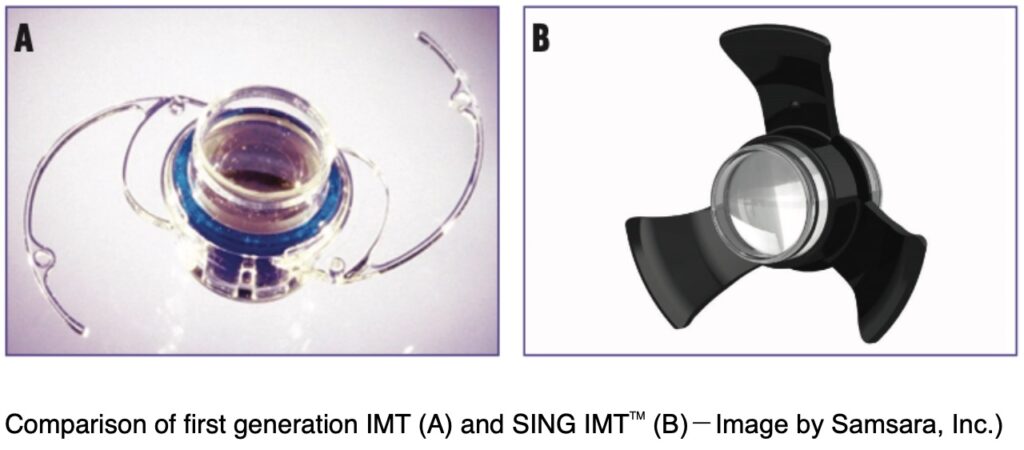
The SING IMTTM is approved in several countries, but not yet in the United States. Samsara has received FDA approval to initiate a U.S.-based clinical trial to evaluate improvements in sharpness of vision and to ensure safety of the device in people living with late-stage age-related macular degeneration. The CONCERTO clinical trial is now recruiting participants.
Participants in the clinical trial will receive a SING IMTTM in one eye. They must be:
- Aged 65 or older with late-stage AMD in both eyes
- Cannot have had previous cataract surgery in the study eye
- Must agree to rehabilitation and training after receiving the IMT (Up to 10 vision rehabilitation visits scheduled over 12 months to learn how to best use the implanted device as well as daily at-home exercises)
Both the treated and the non-study eye will be examined in five visits over 12 – 15 months with a study eye care provider. You will also have visual training and assessments with a low vision specialist before surgery to determine if there is improvement in your vision with the use of a telescope and if you would be a good candidate for the investigational implanted device. The CONCERTO clinical trial will take place in up to twenty U.S. clinical sites, currently enrolling in California, Florida, Massachusetts, New Jersey, Pennsylvania, and Wisconsin. Follow this link for complete details about the trial and locations.
The SING IMTTM is not a cure for late-stage dry (geographic) AMD. It will not return vision to the level a patient had before AMD, and it will not completely make up for vision loss. The ability to ambulate (walk around) after receiving the SING IMTTM will be almost entirely dependent on the peripheral vision in the non-surgical eye. The established IMT technology has shown improvements in visual acuity and visual function and quality of life for individuals with permanent vision loss due to AMD (more than 90% of implanted eyes experienced more than 2 lines of visual acuity gained, and the improvements were sustained for up to 5 years follow-up). (2)
To learn more about the study and inquire about participation, visit www.concertostudy.com.
_______________________________________
1. In addition to the 217 patients implanted during the pivotal trial, once FDA marketing authorization was received, and CE marketing outside of the US was granted, the device has also been implanted in 400+ patients commercially to reach more than 600 patients.
2. Hudson HL, Lane SS, Heier JS, et al; IMT-002 Study Group. Implantable miniature telescope for the treatment of visual acuity loss resulting from end-stage age-related macular degeneration: 1-year results. Ophthalmology. 2006;113(11):1987-2001.
________________________________________
Author:
Dan Roberts, Editor
Living Well With Low Vision